In their latest blog piece, Cerebral Palsy Alliance Research Institute’s Dr Madison Paton and Dr Megan Finch-Edmondson explore just what we’ve learned from 15 years of stem cell studies involving people living with cerebral palsy – and where to next.
Our team recently summarised all available human research to find out how many people with cerebral palsy have been treated with stem cells. Incredibly, we found that more than 2,400 people have been treated since 2004. With all this information, what have we learnt, and where to next?
What have we learnt?
Research into other stem cell types are progressing. This includes mesenchymal stem cells, haematopoietic or blood-forming stem cells, and neural (brain) and neural-like stem cells.
Whilst evidence is mounting, no stem cell treatment for cerebral palsy is approved for use anywhere in the world.
The majority of research is still in early Phase I (i.e. is it safe?) or Phase II (i.e. is it worthwhile?) studies.
More research is required.
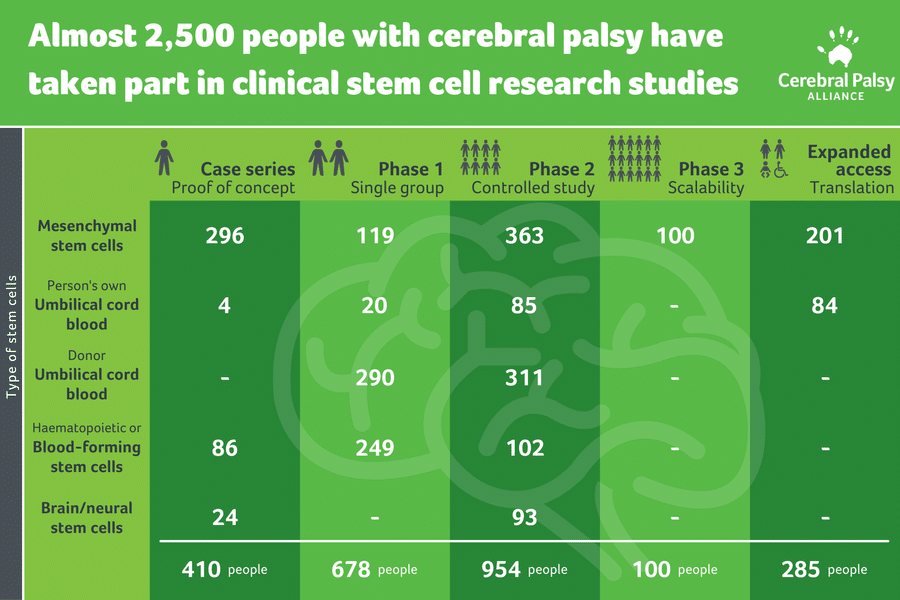
Figure 1: Number of participants treated with cerebral palsy over 15 years of stem cell research
adapted from Paton, M.C., Finch-Edmondson, M., et al (2021)
Where to next?
A missing piece to progress stem cell treatments for cerebral palsy are Phase III clinical trials. Phase III clinical trials help us to answer, if we treat an even larger group of people, can we still show safety and effectiveness? You can learn more about the research pipeline and trial phases here.
We believe the time is right for a Phase III trial using umbilical cord blood. This research will help us understand if umbilical cord blood is effective in improving movement for those with cerebral palsy in a large population. A Phase III trial is the missing piece to enable regulatory approval and access to stem cell treatment, both overseas and in Australia.
Whilst we believe that umbilical cord blood is ready for large Phase III trials, others stem cell types need to first move through earlier phases of the clinical trial pipeline. This will help to answer questions such as “how does it work?”, “what is the best dose?”, “how can we give the treatment?”, and “when do we give the treatment?”. These unanswered questions now shape the field’s future research directions.
You can learn more about the 15 years of stem cell research for cerebral palsy in our recent publication:
Reference: Paton, M.C., Finch-Edmondson, M., Fahey, M.C., London, J., Badawi, N. and Novak, I. (2021), Fifteen years of human research using stem cells for cerebral palsy: A review of the research landscape. J Paediatr Child Health, 57: 295-296.
To read more about stem cells and cerebral palsy research, read Madison and Megan’s previous blog posts: