
Written by Dr Madison Paton and Dr Megan Finch-Edmondson
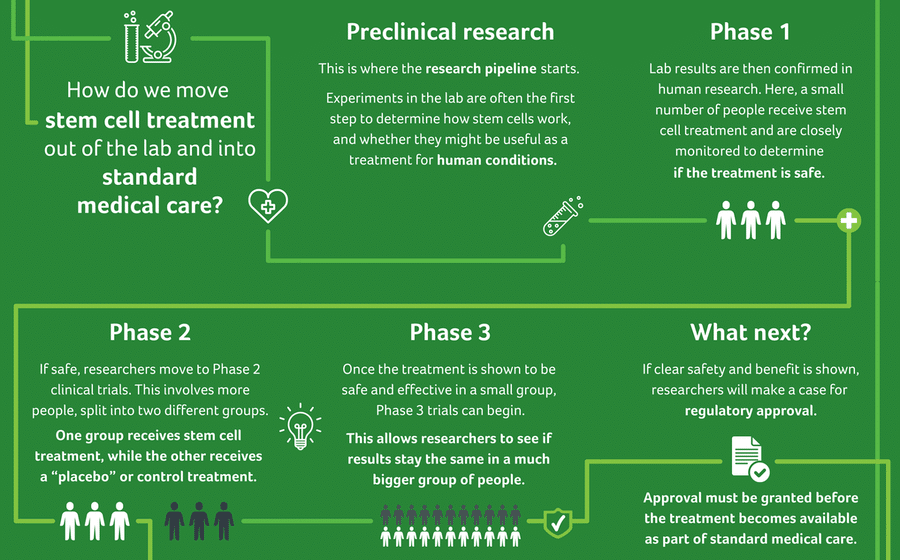
Before a new treatment is approved for standard medical care, there is often years of research and experiments conducted in a lab. These experiments are key to developing safe and useful stem cell treatments. For instance, if we want to know whether a certain stem cell may be beneficial for treating brain injury, we must first look at what that stem cell might be capable of.
In the lab, we carry out a series of experiments to understand what stem cells look like, how they work, what environments work best for them, if they can copy themselves, generate other cells and tissues, and so much more. After this, we then need to investigate whether a stem cell treatment behaves similarly in a ‘living system’ – in animals, for example. This is known as pre-clinical research.
Pre-clinical research involves the use of small (rats, mice and rabbits) and/or larger animals (sheep, pigs and monkeys) in studies that help to identify the safety, benefit or mechanism of a treatment. Studies involving animals must undergo rigorous ethical review and are carefully monitored to ensure they follow the code for the care and use of animals for scientific purposes. Through these studies, we can understand how stem cells may act in a living system to help repair the brain after injury.
While pre-clinical research can provide information about the potential usefulness of a treatment, it’s very important that we confirm the same results in humans. That’s why the next step involves research with human volunteers. This is referred to as a clinical trial.
Clinical trials help determine if a particular treatment is safe and effective for use in a specific condition or population. New knowledge learned through lab work and pre-clinical trials, informs how the research should be conducted with humans.
So, what do clinical trials in Australia look like?
Phase 1
The first stage is a called a “Phase 1” clinical trial. A small number of people receive the treatment and are closely monitored to determine if it is safe. For cerebral palsy, there have been more than 20 Phase 1 trials completed across a number of stem cell treatments so far.
Phase 2
Once Phase 1 is complete, researchers can move to a Phase 2 clinical trial. This involves a larger number of people who are split into two different groups. One group receives the treatment while the other receives a “placebo” or control treatment. The outcomes of the two groups are then compared. More than 25 Phase 2 trials exploring the use of a variety of stem cell treatments for cerebral palsy have now been completed. A number of Phase 2 clinical trials investigating the use of stem cells in brain injury are also underway.
Phase 3 and beyond
Once Phase 1 and 2 trials have shown a treatment is both safe and effective in a given population, Phase 3 trials can be conducted. Phase 3 trials allow researchers to determine whether the results of earlier trials can also be shown in a much larger population. As yet, there have been no published Phase 3 clinical trials of any stem cell treatments for cerebral palsy. In addition, very few Phase 3 trials are underway. This is now an important area of research focus.
A case for regulatory approval can start to be made once Phase 3 trials are complete – and if the data shows a clear benefit of the treatment. Regulatory approval must be granted before a new treatment can become available as part of standard medical care.
Expanded Access Programs
In some countries, a scheme called ‘Expanded Access‘ allows a small number of people outside of a clinical trial to access an unapproved treatment. This type of scheme is not available in all countries, including Australia, however it has some similarities to our Special Access Scheme. Expanded access has meant some families, in very specific circumstances, can access treatments shown to be effective in earlier stage clinical trials, before they have received regulatory approval.
Do clinical trials interest you?
You can listen to our team discuss how research about stem cells is used to approve new therapies for brain injury in our short FAQ videos here.
To stay up-to-date with clinical trials, including those currently underway, this online database offers the largest registry of clinical trials worldwide.