Umbilical cord blood may be the solution. While access is still limited, progress is underway. In April 2025, the first Australian child was treated under a compassionate access pathway — a landmark moment that signals the beginning of change. Prior to this, access to cord blood was only available via clinical trials. CPA is now leading efforts to expand access for more families
So, what is it, how will people with CP benefit, and what needs to be done to make cord blood treatment a reality for more Australians?
Studies have shown that umbilical cord blood is safe and effective when accompanied with the right therapy. As our clinical knowledge of cord blood treatment evolves, we want to be able to respond appropriately to make cord blood more widely available to children with CP in Australia either via necessary research or compassionate access.

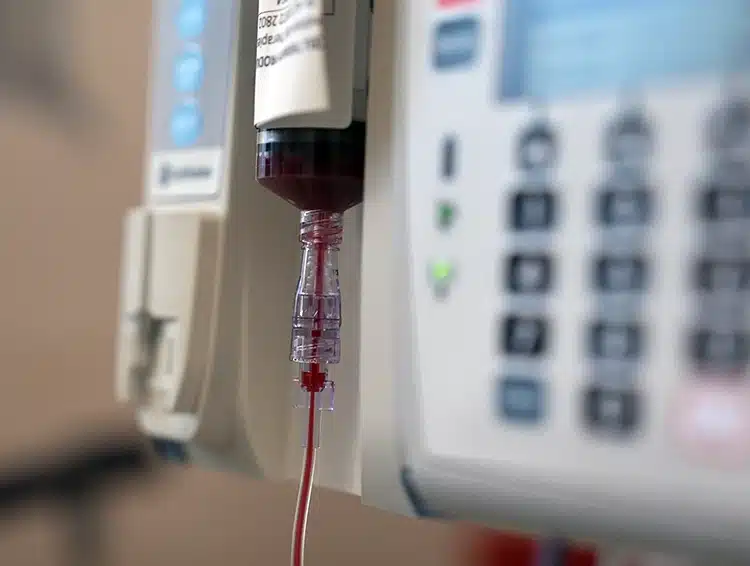
Umbilical cord blood is collected from the placenta after the baby is born and the umbilical cord is cut. Cord blood is a rich source of immune and stem cells, which can activate repair processes in the human body. Long used to treat blood cancers, cord blood has also been studied over the past 20 years as a potential treatment for CP, with promising results in improving brain and motor function.
Cerebral Palsy Alliance researchers are now collaborating with other leaders in the field internationally with the aspiration that, in the future, people with CP may be able to access cord blood as a treatment in Australia. Globally, there have been several studies completed, and the evidence that cord blood can make a tangible positive difference for at least some people with CP continues to grow.
In 2025, we mark five years since CPA first hosted a public forum on the progress of stem cell treatments for the brain. Here, we shared learnings about the communities’ preferences and needs, and called for more research and access to cord blood treatment for CP. Since then, hundreds of children have received regulated cord blood treatment overseas for their CP— but only now is it becoming possible here in Australia.
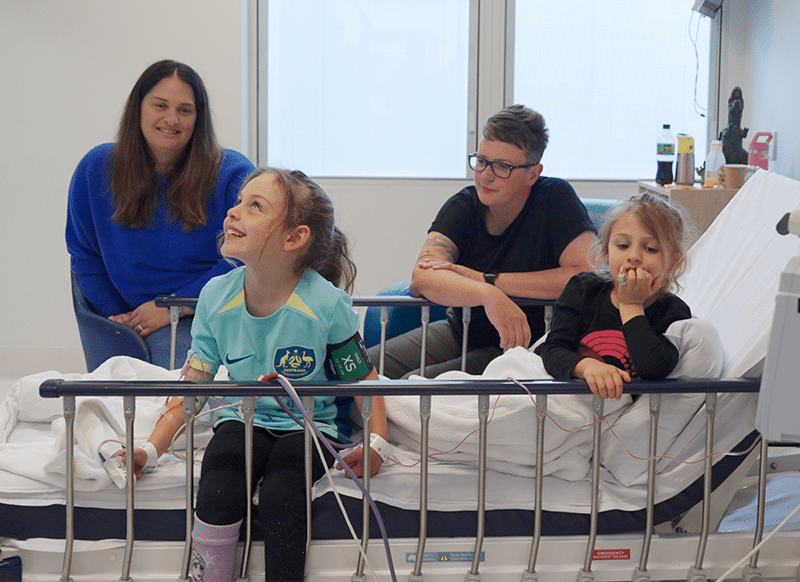
With established safety and growing evidence of efficacy, families travel from around the world to receive regulated cord blood treatment in the US via compassionate access. Madison spent 6 months in the US learning about how access to cord blood is made available, and what we can be doing next.

In April 2025, CPA supported the first Australian family to access cord blood treatment for CP via the Therapeutic Goods Administration (TGA)’s Special Access Scheme (SAS) at Monash Children’s Hospital. This was possible because the child had their own cord blood banked, met participant and treatment eligibility, and was supported by dedicated clinicians and the CPA team.
As stem cell research advances, growing numbers of families are asking why cord blood remains inaccessible in Australia, with many travelling overseas at significant personal expense to access cord blood in other countries.
Unlocking a treatment that can reduce symptom severity for some people with CP could be transformative.

We are coordinating with families, researchers, clinicians and allies to share information and learnings to ensure that this promising treatment can become standard care, ensuring equitable access for all people with CP.
If you are interested in staying up to date, please fill out the form below.
In 2025, a major international research analysis confirmed the safety and potential benefit of cord blood treatment for some children with CP from data contributed from around the world. This research was pivotal to support treatment access. Prior to this, Australia had conducted fundamental research to support the safety of sibling cord blood in children with CP. However, this research only enrolled a small and select group of participants, and since, research overseas had been expedited.
In 2025, around the same time as our up-to-date analysis of global data was published, Australia’s first treatment under compassionate access took place — backed by CPA, clinicians, and new research insights
Together, these breakthroughs mark a shift: this is no longer just emerging science — it’s an access issue. CPA is stepping up to lead this change.


CPA is supporting families and clinicians to take the next steps toward fair and safe access to cord blood treatment in Australia. By registering your details below, you’ll be kept informed about new developments — and you’ll help us understand and advocate for what matters most to families.
"*" indicates required fields
Much like CP research, cell therapy research including cord blood treatment is an emerging area of interest. Below, our researchers have outlined some of the most frequently asked questions from parents and people with CP.
Umbilical cord blood is collected from the placenta after the baby is born and the umbilical cord is cut. To ensure quality and safety, cord blood cells must be collected and frozen by a certified public or private cord blood bank.
Umbilical cord blood is not yet an approved treatment for cerebral palsy (CP) anywhere in the world. However, in some cases, it may be accessed through ‘compassionate access’ pathways.
Cord blood has been studied as a potential treatment for CP for over 20 years, with more than 800 children and adults treated in clinical trials. These studies have consistently shown that cord blood is safe, and several have reported improvements in motor skills.
A new publication brings together the findings from all these studies. Read that paper here.
Compassionate access (also called expanded access) allows a person to receive a treatment before it is fully approved, or to access an approved treatment when they don’t meet the usual eligibility criteria, but their doctor believes it may help. For children with cerebral palsy, compassionate access to cord blood is being considered because safety has been demonstrated, and clinical trials data shows potential benefits for movement skills. In Australia, this is possible through the Therapeutic Goods Administration (TGA)’s Special Access Scheme.
More clinical trials are needed to answer important questions—like how to improve access to donated cord blood, and whether cord blood can support areas of development beyond movement, such as thinking skills and quality of life, which we know matter deeply to families.
Umbilical cord blood treatment is given via a drip into the arm. This takes approximately 20 minutes and is followed by monitoring for any side effects over a few hours. Medications such as antihistamines and anti-inflammatory drugs may be given just prior to the treatment which can help to reduce any potential side effects.
In the lead up to treatment, a suitable umbilical cord blood unit must be identified and assessed for the number of cells and the quality of the unit. The frozen cord blood cells are carefully transported and handed over to the hospital where treatment is provided to make sure they are in the best possible condition for infusion. Cord blood as a treatment should be coupled with physiotherapy or occupational therapy after infusion.
Cord blood is a mixed cell therapy, meaning that it contains many different types of cells that could be useful in the treatment of CP. These includes immune, stem and progenitor cells and research demonstrates these cells release factors and influence their environment to support brain repair. Cord blood cells don’t need to reach the brain directly to have their effects on the brain.
In research studies, cord blood has been shown to improve gross motor skills (such as walking), especially in younger children with milder CP. In some research, cord blood treatment improved other outcomes such as fine motor skills and cognition however more research would be required to understand the potential of cord blood for improving these other outcomes.
Umbilical cord blood treatment should be given in a hospital or clinic setting under the supervision of a doctor with training in handling and giving blood or cell products (e.g., a haematologist or infusionist). This is because there is a small risk of a reaction occurring during the treatment that may require immediate medical treatment.
The doctor will also be required to have access to, or receive, banked umbilical cord blood and a co-located laboratory facility to manage the thawing process safely.
Research studies in cerebral palsy (CP) have used umbilical cord blood from three sources: the child themselves (autologous), a sibling, or an unrelated donor (allogeneic). All three sources have been shown to be safe, and each has demonstrated potential benefits for children with CP.
In Australia, both private and public cord blood banks exist. The public bank stores donated cord blood for use in transplants or approved research, while the private bank stores cord blood collected for personal or family use—such as a child’s own cord blood or that of a sibling.
Most families do not have their own child’s cord blood stored or access to a sibling unit, so would require a donor. However, donor cord blood from the Australian public bank can only be released for use in research—not for individual treatment. In contrast, compassionate access in Australia has been granted in some cases using a child’s own cord blood stored in the private bank.
Whilst widespread access to this treatment is not currently available, progress is being made. In early 2025, the first child in Australia was treated with their own cord blood via compassionate access. CPA is now helping other families explore this pathway.
Cord blood treatment is not yet approved for CP. Publicly banked cord blood units are only available for use via clinical trials or for approved conditions as part of standard clinical care. In Australia we have limited access mechanisms for CP and some people seek treatment overseas via compassionate access. Read more information here
Umbilical cord blood treatment has been given to children and even adults with all types and functional levels of CP in research settings.
The most recent research shows that cord blood is most helpful at improving gross motor skills in younger children (under 5 years), and with milder CP.
There is limited research evidence to suggest that umbilical cord blood may improve cognition in children with CP. It is not known whether umbilical cord blood can be effective for improving other skills/goals in older children and adults, or in those with more severe CP.
Research shows that treating with a higher number of cord blood cells is better for improving gross motor skills. Whilst the exact number of cells needed is not known, research suggests that doses over 50 million cells per kilogram (body weight) per treatment, may produce the best results, but higher doses seem even better.
While not yet confirmed in human studies, repeated treatments are believed to offer added benefits. however, repeat doses are also not yet approved anywhere in the world.
While research tells us that cord blood treatment for CP is safe, cord blood treatment can still carry risks, like any medical procedure.
The main risks of cord blood infusions are:
There are several cell therapy interventions being investigated for the treatment of CP. Amongst all cell therapies, umbilical cord blood has the best research evidence. In addition to cell therapies, there are several other approaches that may help to improve gross motor skills or other outcomes.
We are committed to establishing a participant registry where we collect important outcome data that we can reflect on as more people are treated in Australia. Overseas, other compassionate access programs collect necessary safety follow up but are often under-resourced to collect information to assess if the patient has improved. Registries help to support us in improving this landscape and collect invaluable information that contributes to our understanding of treatment benefit. Ultimately this information over time can support what we know about cord blood treatment and be used to gather more resourcing to provide care as well as work towards overall treatment approval.
It is thought that umbilical cord blood treatment may help to provide a boost to the effects of rehabilitation for improving gross motor skills, and other outcomes. Cord blood may help the brain form new pathways, while rehabilitation strengthens and refines them. It is therefore recommended that a training intervention (e.g., rehabilitation), directed at the child’s goals, is done following cord blood treatment. Best practice guidelines indicate 14-40 hours of goal-directed training within a 6-week period.
Discover our progress and achievements through time

World leading clinician researcher Professor Novak spearheads a global cell therapies network for her 2013 Fulbright Fellowship. In 2016, the first child with CP was treated with sibling cord blood in the SCUBI-CP Trial. In 2018, the first Stem Cell Reference Group meeting was held at CPA, facilitated by the Regeneration Theme.
Summary of evidence for all CP interventions shows cord blood is a "green light" intervention- one of 54 - but the only to not be available as part of standard care. CPA Public Forum held that successfully gathered support for cell therapies and Claire's story spotlighted to show one family's journey of cord blood access via research.
2021 marks 15 years of research on cell therapies for cerebral palsy. CPA launches Communicating Research Series to bring science to the public and share important updates.

Australia’s first trial of sibling cord blood for CP published (SCUBI-CP) showing that treatment was safe. Meanwhile, evidence of treatment benefit is building as the seventh randomised Phase II trial is published overseas (Sun et al. 2022).

CPA Regeneration Theme Leader Dr Megan Finch-Edmondson presents preliminary results of the world’s largest data analysis of cord blood for CP (called an IPDMA), winning Best Abstract at Cord Blood Connect, USA. Regeneration Theme Research Fellow Dr Madison Paton wins Australasian Society for Stem Cell Research Rising Star Award.

The Therapeutic Goods Administration approves first Australian to receive cord blood for CP compassionately. Dr Paton receives Fullbright Fellowship at Duke University to advance access to cord blood treatment for CP.

Zara becomes the first Australian with CP to receive her own cord blood. The infusion happened at Monash Children's Hospital in the same month that the IPDMA is published to demonstrate the safety and benefit of cord blood in some children with CP.

The case report about a 6-year-old patient with CP who compassionately received an infusion of their own cord blood was published in the Journal of Paediatrics and Child Health in early January. This demonstrated the safety and feasibility of autologous cord blood infusions in Australia and explored the ethical and translational considerations. Read more here

CPA also hosts a Stem Cell Reference Group that meets twice a year. The purpose of the group is to provide the perspective of people with CP and their families to shape decisions about research priorities, specific research questions and the design of new stem cell research projects. This group is also helping shape how access can expand fairly and ethically for children who may benefit from cord blood treatment.
Interested in joining? Please email celltherapies@cerebralpalsy.org.au
We can be contacted via celltherapies@cerebralpalsy.org.au
Help transform lives by supporting cerebral palsy research. Your donation brings hope and progress to those in need.